Volume
of NaOH (ml)
|
pH
of Acetic Acid
|
1
|
3.54
|
2
|
3.61
|
3
|
3.87
|
4
|
4.00
|
5
|
4.10
|
6
|
4.20
|
7
|
4.33
|
8
|
4.45
|
9
|
4.45
|
10
|
4.55
|
11
|
4.63
|
12
|
4.68
|
13
|
4.74
|
14
|
4.76
|
15
|
4.80
|
16
|
4.92
|
17
|
5.00
|
18
|
5.14
|
19
|
5.26
|
20
|
5.35
|
21
|
5.50
|
22
|
5.66
|
23
|
5.92
|
24
|
6.59
|
25
|
10.14
|
Table 1 : pH and volume of NaOH added to Acetic acid.
Volume
of NaOH (ml)
|
pH
of Phosphoric Acid
|
pH
of Amino Glysine Acid
|
3
|
2.08
|
8.89
|
6
|
2.10
|
9.30
|
9
|
2.17
|
9.60
|
12
|
2.37
|
9.80
|
15
|
2.49
|
10.00
|
18
|
2.68
|
10.22
|
21
|
3.12
|
10.46
|
24
|
4.89
|
10.78
|
27
|
6.11
|
11.33
|
30
|
6.50
|
11.87
|
33
|
6.75
|
12.08
|
36
|
7.09
|
12.23
|
39
|
7.35
|
12.34
|
42
|
7.69
|
12.42
|
45
|
8.54
|
12.48
|
48
|
10.60
|
12.53
|
51
|
11.32
|
12.57
|
54
|
11.69
|
12.62
|
57
|
11.87
|
12.64
|
60
|
12.01
|
12.67
|
63
|
12.14
|
12.69
|
66
|
12.23
|
12.70
|
69
|
12.32
|
12.71
|
72
|
12.37
|
12.75
|
75
|
12.41
|
-
|
78
|
12.47
|
-
|
81
|
12.50
|
-
|
84
|
12.53
|
-
|
87
|
12.55
|
-
|
90
|
12.57
|
-
|
93
|
12.60
|
-
|
96
|
12.62
|
-
|
99
|
12.64
|
-
|
102
|
12.65
|
-
|
105
|
12.68
|
-
|
108
|
12.70
|
-
|
111
|
12.70
|
-
|
114
|
12.70
|
-
|
117
|
12.72
|
-
|
120
|
12.76
|
-
|
123
|
12.77
|
-
|
126
|
12.84
|
-
|
129
|
12.87
|
-
|
132
|
12.88
|
-
|
135
|
12.88
|
-
|
138
|
12.88
|
-
|
141
|
12.91
|
-
|
145
|
12.92
|
-
|
148
|
12.93
|
-
|
Table 2 : pH and Volume of NaOH added to phosphoric acid and amino glycine acid.
 |
| Graph 1 : Acetic acid is titrated with NaOH |
CH3COOH (aq) + NaOH (aq) à CH3COONa (aq) + H2O (l)
The start of the grapf shows a relatively rapid rise in pH but this slows down as a buffer solution containing ethanoic acid and sodium ethanoate is produced at the 8 ml and 9 ml. The pH does not change too much until it get close to the equivalent point. The average reading for the pH of acetic acid is 4.97. pKa values are generally determined by titration. A carefully calibrated, automated, recording titrator is used, the free acid of the material to be measured is titrated with a suitable base, and the titration curve is recorded. How to determine the pKa of this titration? Let’s look at the chemical equation of acetic acid dissociation and the calculation related below:
CH3COOH (aq) à CH3COO- (aq) + H+ (aq)
pH = pKa + log [ A- ] / [ HA]
4.45 = pKa + log (0.1 M / 0.1 M)
pKa = 4.45
|
Therefore, we have known that the pKa of the titration of acetic acid with sodium hydroxide is 4.45. We have to choose the point of inflection at the graph because the inflection point indicates the pKa value. The graph of acetic acid titration is differs from other acid because acetic acid is monoprotic acid. Hence, it produces only one inflection point due to the dissociation of hydrogen ion. The inflection point also well known as midpoint because of the buffer system formed in this titration that function to maintain the pH of the mixed solution which is sodium ethanoate. The equivalent point is achieved when enough sodium hydroxide is added to react with all the acetic acid present that exhibits the sharp increase in pH.
_______________________________________________________________________________
The titration of weak acid
(polyprotic acid) with strong base
In
this titration, the weak acid is phosphoric acid with chemical formula,
and the strong base is sodium hydroxide with
the chemical formula, NaOH. We have used the titration method by running the
strong base, sodium hydroxide into the weak acid, phosphoric acid. The plotted
graph and the chemical equation of the reaction as shown below:
The
start of the graph shows a relatively rapid rise in pH but this slows down as a
buffer solution containing phosphate acid and sodium phosphoate is produced at
the pH 2.08, 7.40 and 12.70. The pH does not change too much until it get close
to the equivalent point. The average reading for the pH of phosphoric acid is 10.13.
pKa values are generally determined by titration. A carefully calibrated,
automated, recording titrator is used, the free acid of the material to be
measured is titrated with a suitable base, and the titration curve is recorded.
How to determine the pKa of this titration? Let’s look at the chemical equation
of acetic acid dissociation and the calculation related below:
H3PO4 (aq) à
H2PO4 – (aq) + H+ (aq)
pH = pKa + log [
A- ] / [ HA]
2.08 = pKa + log (0.1 M / 0.1 M)
pKa = 2.08 (1st inflection point )
H3PO4 (aq) à H2PO4 – (aq) + H+ (aq)
pH = pKa + log [ A- ] / [ HA]
7.40 = pKa + log (0.1 M / 0.1 M)
pKa = 7.40 (2nd inflection point )
H3PO4 (aq) à H2PO4 – (aq) + H+ (aq)
pH = pKa + log [ A- ] / [ HA]
12.70 = pKa + log (0.1 M / 0.1 M)
pKa = 12.70 (3rd inflection point )
Therefore,
we have known that the pKa of the titration of phosphoric acid with sodium
hydroxide is 2.08 , 7.40 and 12.70. We have to choose the point of inflection at the graph
because the inflection point indicates the pKa value. Based on the
Henderson-Hasselbalch relationship indicates that the pH of the buffer solution
does not depend on the total concentration of the buffering acid and conjugate
base, but only on the pKa and ration of the concentration of these two species.
The graph of phosphoric acid titration is differs from other acid because
phosphoric acid is polyprotic acid that contains more than one acidic hydrogen.
Hence, it produces two inflection point due to the dissociation of hydrogen ion
in this titration. The inflection point also well known as midpoint because of
the buffer system formed in this titration that function to maintain the pH of
the mixed solution which is sodium phosphoate. The equivalent point is achieved
when enough sodium hydroxide is added to react with all the phosphoric acid
present that exhibits the sharp increase in pH.
________________________________________________________________________________
For
simple amino acids, like glycine, have two dissociation steps: (1) the loss of
H+ from the acidic carboxyl group at low pH; and (2) the loss of H+ from the
more basic amino group at high pH. The pKa value for each dissociable group of
an amino acid can be determined from such a titration curve by extrapolating
the midpoint of each buffering region (the plateau) within the curve. Also
revealed from the diagram is a point on the curve where the amino acid behaves
as a neutral salt. Specifically, this point is known as the isoelectric point
(pI), and is loosely defined as the pH where the amino acid is predominantly a
zwitterion. Furthermore, the pI can be approximated as halfway between the two
points of strongest buffering capacity and can be estimated by:
pI = 1/2 ( pK1 + pK2)
where
K1 and K2 are the dissociation constants for the deprotonation of glycine’s
carboxylic acid and amino groups.
Added Information

Added Information
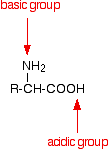
Zwitterions in simple amino acid solutions
An amino acid has both a basic amine group and an acidic carboxylic acid group.

There is an internal transfer of a hydrogen ion from the -COOH group to the -NH2 group to leave an ion with both a negative charge and a positive charge.
This is called a zwitterion.

This is the form that amino acids exist in even in the solid state. If you dissolve the amino acid in water, a simple solution also contains this ion.
A zwitterion is a compound with no overall electrical charge, but which contains separate parts which are positively and negatively charged.



No comments:
Post a Comment